Oxidative Addition of Methyl Iodide to [RhI(CO){Ph2PCH2P(S)Ph2}]
6. X-ray Crystal Structure of [RhI2(COMe)(dppms)], 3.
The structure of the acetyl complex, [RhI2(COMe)(dppms)] 3 is shown below. (Jmol) Hydrogen atoms are omitted for clarity.
The molecule has a distorted square pyramidal geometry, with one of the two iodide ligands distinctly below the basal plane.
Two static views of the molecular structure are shown below.
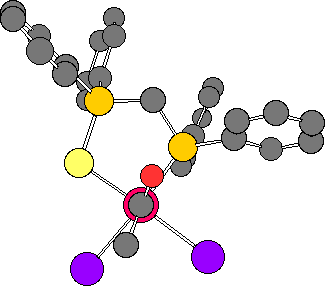
In this view, looking down the MeC(O)-Rh bond, the conformation of the acetyl ligand relative to the rest of the coordination sphere, can be seen. The plane of the acetyl group lies so that steric interactions with the phenyl groups of the dppms ligand are minimised.
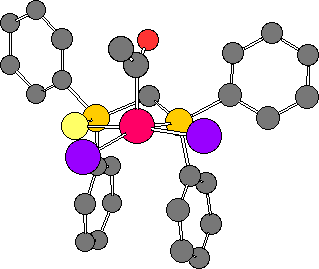
This "twisting" of the acetyl ligand forces the methyl to lie almost "eclipsed" with one of the iodide ligands. It appears that the effect of this is to "push" the iodide ligand below the basal plane of the square pyramid.
Structures have been determined for the closely related diphosphine complexes, [RhI2(COMe)(dppm)] [Ref. 7] and [RhI2(COMe)(dppp)] [Ref. 8]. The dppp complex shows a "twisted" acetyl conformation and distorted square pyramidal structure, similar to that found for 3. The dppm complex, by contrast, shows a more symmetrical structure with the plane of the acetyl ligand bisecting the I-Rh-I angle.
Go to Next Page (Summary)
Go to Poster Index


