Oxidative Addition of Methyl Iodide to [RhI(CO){Ph2PCH2P(S)Ph2}]
2. Catalytic Cycle
The catalytic cycle proposed by researchers at BP Chemicals is shown below:
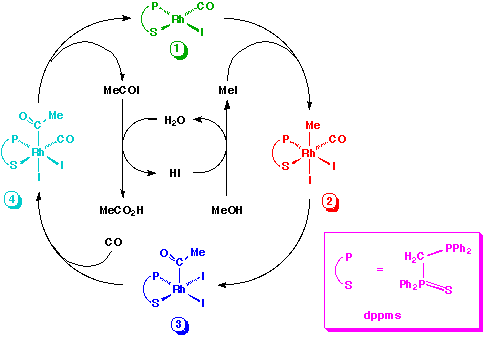
Complexes 1, 3 and 4 were isolated or observed spectroscopically and an X-ray structure was obtained for the chloride analogue of 1. Complex 4 was observed as a mixture of two isomers.
The only Rh-carbonyl species observed under catalytic conditions was complex 1. The rate of catalytic carbonylation was found to be first order in [MeI] and independent of CO pressure above a threshold value. It was therefore suggested that the rate determining step of the catalytic cycle is oxidative addition of MeI to 1, similar to the conventional process.
In this poster, we report a kinetic investigation of the key oxidative addition reaction of 1 with methyl iodide. We present preliminary spectroscopic evidence for the reactive rhodium methyl intermediate, 2, and we compare our data with the conventional (non-phosphine promoted) catalyst. We also report the X-ray crystal structure of the rhodium acetyl complex 3.
Go to Next Page (Infrared Kinetics Experiments)
Go to Poster Index

