Oxidative Addition of Methyl Iodide to [RhI(CO){Ph2PCH2P(S)Ph2}]
3. Reaction Kinetics - Infrared Spectroscopy
A series of spectra recorded during the reaction of 1 with methyl iodide (1.6 M) in CH2Cl2 is shown below.
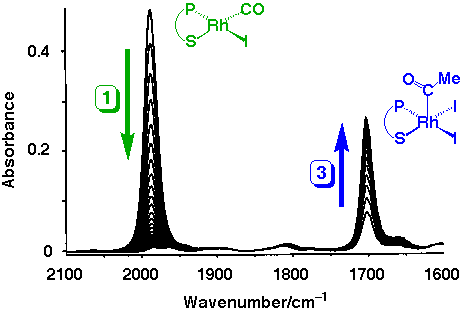
The single terminal n(CO) band of 1 at 1987 cm-1 decays and is replaced by an absorption at 1701 cm-1 due to the acetyl group of 3.
The clean formation of 3 from 1 suggests that slow, rate determining oxidative addition of methyl iodide is followed by rapid migratory CO insertion.
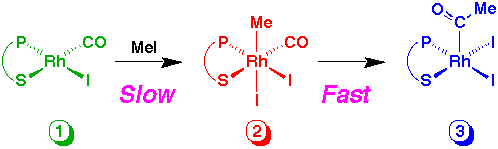
By adjusting the reaction conditions we have found spectroscopic evidence for the rhodium methyl intermediate, 2, present at low concentration. To see the spectra Click Here
Kinetic Analysis
The graph below shows absorbance vs. time plots for the bands of 1 and 3
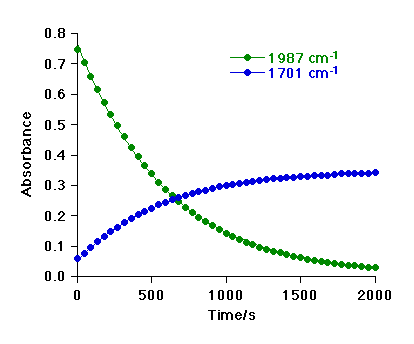
The decay of 1 is exponential, showing that the reaction is first-order in [1].
Analysis of the data therefore gives a pseudo-first order rate constant (kobs) for each kinetic run.
Values of kobs were obtained as a function of [MeI] and temperature.
Go to Next Page (Kinetic Data)
Go to Poster Index



