Oxidative Addition of Methyl Iodide to [RhI(CO){Ph2PCH2P(S)Ph2}]
1. Introduction
Methanol carbonylation is one of the most important examples of homogeneous transition metal catalysis. More than 5 millon tonnes of acetic acid are made annually using the process. For many years, an iodide promoted rhodium catalyst has been preferred. The active species is believed to be a square planar Rh(I) complex, cis-[Rh(CO)2I2]- [Ref. 1].
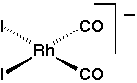
Oxidative addition of methyl iodide to this complex is the rate determining step for catalysis. The mechanism for oxidative addition is thought to involve an SN2-type nucleophilic attack by the metal centre at the carbon of methyl iodide [Ref. 2]. One approach to increase catalytic activity is therefore to increase the nucleophilicity of rhodium complex by modification of the coordination sphere. This strategy has been shown to be valid by the increased activity found on using phosphine ligands such as Ph2PCH2CH2P(O)Ph2 [Ref. 3].
Recent results from researchers at BP Chemicals showed that a mixed phosphorus-sulfur bidentate ligand, bis(diphenylphosphino)methane sulfide (dppms) also gives a highly active catalyst [Ref. 4].

It was found that carbonylation rates were improved by a factor of 6 - 9 by the dppms ligand.
Synthetic and spectroscopic studies led to the proposal of a catalytic cycle.
Go to Next Page (Catalytic Cycle)
Go to Poster Index


