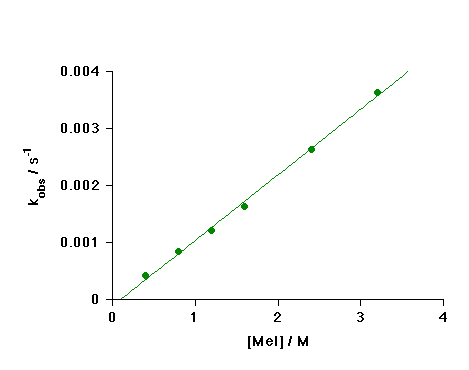
Dependence on Methyl Iodide Concentration
The graph below shows values of the observed pseudo-first-order rate constant, kobs, plotted against methyl iodide concentration (25 oC, CH2Cl2).
The linear plot shows the reaction is first order in [MeI] and therefore second-order overall:
Rate = k2[1][MeI]
and kobs = k2[MeI]
The value of k2 obtained from the slope of this plot is 1.1 x 10-4 M-1 s -1. This rate constant is ca. 40 times larger than that measured for [RhI2(CO)2]- [Ref. 5].
Temperature Dependence
Measurement of k2 over the temperature range 10 - 35 oC allowed an Eyring plot to be constructed:
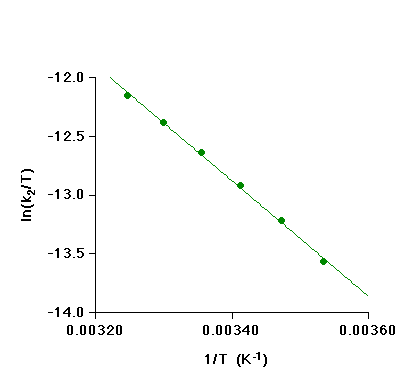
The enthalpy and entropy of activation can be calculated from the slope and intercept of this plot. In the table below, the resulting values are compared with activation parameters for the reaction of [RhI2(CO)2]- with MeI [Ref. 5].
| Complex | DH* / kJ mol-1 | DS* / J mol-1 K-1 | k185 (calc) /M-1 s-1 |
| [RhI(CO)(dppms)] (1) | 41 (± 1) | -166 (± 4) | 0.43 |
| [RhI2(CO)2]- | 50 (± 1) | -165 (± 4) | 0.046 |
Using these activation parameters we can extrapolate to higher temperature to obtain a comparison of the two complexes closer to catalytic conditions. The predicted rates suggest that [RhI(CO)(dppms)] (1) would react with MeI ca. 10x faster than [RhI2(CO)2]- at 185 oC. This predicted acceleration matches well the 6 - 9 fold enhancement observed in catalytic experiments [Ref. 4]. Our kinetic studies therefore provide an excellent model for the catalytic systems.